When conducting a chemical reaction, it is crucial to understand the concept of limiting reactants and percent yield. These concepts play a significant role in determining the efficiency and effectiveness of a reaction. A limiting reactant is the reactant that limits the amount of product that can be formed, while percent yield is a measure of the efficiency of a reaction. By completing a worksheet on limiting reactant and percent yield, students can practice applying these concepts and enhance their understanding of stoichiometry.
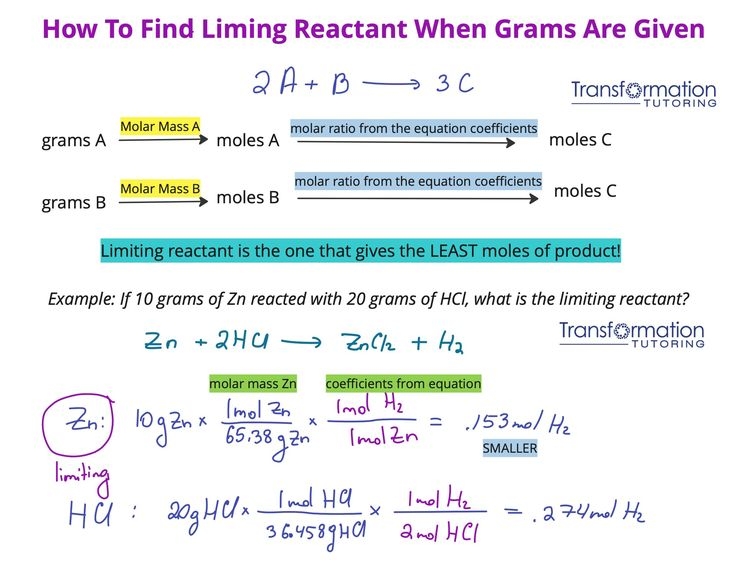
Limiting reactant and percent yield worksheets typically consist of a series of chemical reactions where students are asked to determine the limiting reactant, calculate the theoretical yield, and then compare it to the actual yield to calculate the percent yield. These problems require students to apply stoichiometry principles and mathematical calculations to determine the most efficient reactant and the amount of product that can be obtained.
One common type of problem found on these worksheets involves determining the limiting reactant in a reaction between two or more reactants. Students must first balance the chemical equation and then use stoichiometry to calculate the amount of product that can be formed from each reactant. The reactant that produces the least amount of product is the limiting reactant, as it determines the maximum amount of product that can be obtained.
Another type of problem on limiting reactant and percent yield worksheets involves calculating the percent yield of a reaction. Students are given the actual amount of product obtained from a reaction and the theoretical yield calculated from stoichiometry. By dividing the actual yield by the theoretical yield and multiplying by 100, students can determine the percent yield of the reaction. This calculation provides insight into the efficiency of the reaction and allows students to assess the factors that may have impacted the yield.
In conclusion, limiting reactant and percent yield worksheets are valuable tools for students to practice applying stoichiometry principles and understanding the efficiency of chemical reactions. By completing these problems, students can enhance their problem-solving skills and gain a deeper understanding of the relationships between reactants and products in a chemical reaction. Through practice and repetition, students can improve their proficiency in determining limiting reactants and calculating percent yield, ultimately strengthening their grasp of stoichiometry concepts.