Charles Law is a fundamental principle in the field of chemistry that describes how gases tend to expand when heated. This law is named after Jacques Charles, a French scientist who first formulated it in the late 18th century. Understanding Charles Law is essential for students studying chemistry, as it helps them comprehend the behavior of gases under different temperature conditions.
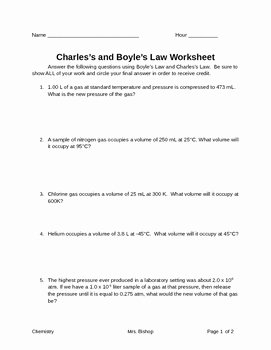
One way to reinforce the concept of Charles Law is by using a worksheet. A Charles Law worksheet typically consists of a series of problems or questions that require students to apply the principles of Charles Law to solve them. These worksheets are valuable tools for practicing calculations and understanding the relationships between temperature and volume in gases.
Charles Law Worksheet
When working on a Charles Law worksheet, students are often asked to calculate the final volume of a gas at a given temperature, based on its initial volume and temperature. They may also be required to determine the temperature at which a gas will reach a specific volume, given its initial volume and temperature. These problems help students develop critical thinking skills and apply the principles of Charles Law in real-world scenarios.
Furthermore, Charles Law worksheets may include questions that require students to graph the relationship between temperature and volume for a gas, demonstrating how the volume of a gas changes as temperature increases or decreases. This visual representation can enhance students’ understanding of the concept and help them visualize the relationship between temperature and volume in gases.
By completing a Charles Law worksheet, students can practice applying the principles of Charles Law and solidify their understanding of the relationship between temperature and volume in gases. These worksheets provide a hands-on approach to learning and allow students to test their knowledge and skills in solving problems related to Charles Law.
In conclusion, a Charles Law worksheet is a valuable tool for students studying chemistry to reinforce their understanding of the principles of Charles Law. By working through problems and questions related to temperature and volume in gases, students can improve their problem-solving skills and enhance their comprehension of this fundamental concept in chemistry.