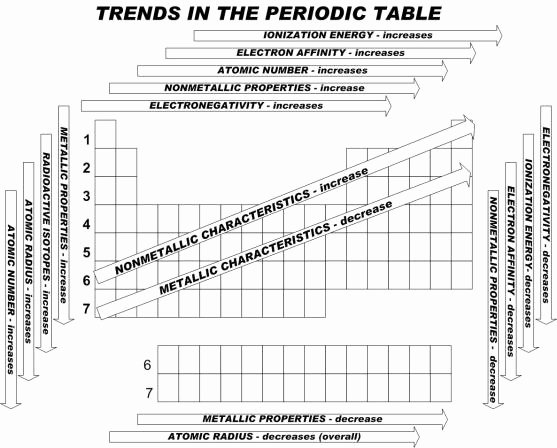
Understanding periodic trends in the periodic table is crucial for predicting the behavior of elements and their properties. These trends provide valuable insights into the periodicity of elements and help us make predictions about their chemical behavior.
One of the most important periodic trends is the atomic radius, which refers to the size of an atom. As you move from left to right across a period in the periodic table, the atomic radius generally decreases. On the other hand, as you move down a group, the atomic radius tends to increase. This worksheet will help you practice identifying and predicting changes in atomic radius based on the position of elements in the periodic table.
Another key periodic trend is electronegativity, which is the tendency of an atom to attract electrons in a chemical bond. Electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. This worksheet will also include questions that will help you understand and predict changes in electronegativity based on the periodic table.
Additionally, the worksheet will cover other periodic trends such as ionization energy, which is the energy required to remove an electron from an atom. Ionization energy tends to increase as you move from left to right across a period and decrease as you move down a group. By practicing problems related to ionization energy, you will gain a better understanding of how this trend varies across the periodic table.
Lastly, the worksheet will touch on trends in electron affinity, which is the energy released when an atom gains an electron. Similar to ionization energy, electron affinity generally increases as you move from left to right across a period and decreases as you move down a group. By completing the exercises on electron affinity, you will be able to make predictions about the behavior of elements in chemical reactions.
Overall, this worksheet on periodic trends will provide you with the practice you need to master these important concepts in chemistry. By understanding how these trends vary across the periodic table, you will be better equipped to make predictions about the properties and behavior of different elements. Practice makes perfect, so be sure to work through each problem carefully to solidify your understanding of periodic trends.